How to repair a damaged heart: key mechanism behind heart regeneration in zebrafish revealed

Cardiovascular diseases, such as heart attacks, are a leading cause of death worldwide resulting from a limited self-healing power of the heart. Unlike humans, zebrafish have the remarkable capacity to recover from cardiac damage. Researchers from the group of Jeroen Bakkers (Hubrecht Institute/ UMC Utrecht) have used the zebrafish to shed light on their regenerative success. They discovered a new mechanism that functions as a switch to push the heart muscle cells to mature in the regeneration process. Importantly, this mechanism was evolutionary conserved as it had a very similar effect on mouse and human heart muscle cells. The results of the study, published in Science on May 18th, show that examining the natural heart regeneration process in zebrafish and applying these discoveries to human heart muscle cells could contribute to the development of new therapies against cardiovascular diseases.
It is estimated that 18 million people die from cardiovascular diseases every year. Many of these deaths are related to heart attacks. In such an event, a blood clot prevents the supply of nutrients and oxygen to parts of the heart. As a result, the heart muscle cells in the obstructed part of the heart die, which eventually leads to heart failure. Although therapies exist that manage the symptoms, there is no treatment that is able to replace the lost tissue with functional, mature heart muscle cells and thereby cure the patients.
Zebrafish as a role model
Unlike humans, some species like zebrafish can regenerate their hearts. Within 90 days after damage, they fully restore their cardiac function. The surviving heart muscle cells are able to divide and produce more cells. This unique feature provides zebrafish hearts with a source of new tissue to replace the lost heart muscle cells. Previous studies successfully identified factors that could stimulate heart muscle cells to divide. Nevertheless, what happens to the newly formed heart muscle cells afterwards had not been studied before. Phong Nguyen, first author of the study, explains: “It is unclear how these cells stop dividing and mature enough so that can they contribute to normal heart function. We were puzzled by the fact that in zebrafish hearts, the newly formed tissue naturally matured and integrated into the existing heart tissue without any problems”.
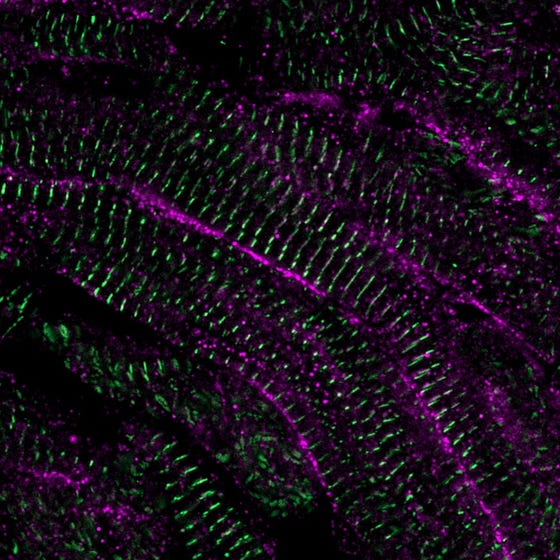
Structured and mature heart muscle cells in the zebrafish heart 60 days after damage. Credit: Phong Nguyen. Copyright: Hubrecht Institute.
LRRC10 drives maturation
To study maturation of the newly formed tissue in detail, the researchers developed a technique for which thick slices of injured zebrafish hearts were cultured outside the body. This allowed them to perform live imaging on the movement of calcium in heart muscle cells. The regulation of calcium moving in and out of heart muscle cells is important for controlling heart contractions and can predict the maturity of the cell. They found that after the heart muscle cells divide, calcium movements changed over time. “The calcium movement in the newly divided cell was initially very similar to embryonic heart muscle cells, but over time the heart muscle cells assumed a mature type of calcium movement. We found that the cardiac dyad, a structure that helped to move calcium within the heart muscle cell, and specifically one of its components, LRRC10, was crucial in deciding whether heart muscle cells divide or progress through maturation. Heart muscle cells that lack LRRC10 continued to divide and remained immature,” says Nguyen.
From fish to human
After Nguyen and his colleagues established the importance of LRRC10 in stopping cell division and initiating maturation of zebrafish heart muscle cells, they moved on to test if their findings could be translated to mammals. To this end, they induced the expression of LRRC10 in mouse and lab-grown human heart muscle cells. Strikingly, LRRC10 changed the calcium handling, reduced cell division and increased the maturation of these cells in a similar manner as observed in zebrafish hearts. Nguyen: “It was exciting to see that the lessons learned from the zebrafish were translatable as this opens new possibilities for the use of LRRC10 in the context of new therapies for patients”.
Clinical impact
The results of the study, published in Science, show that LRRC10 has the potential to drive the maturation of heart muscle cells further through the control of their calcium handling. This could help scientists who are trying to solve the lack of regenerative capacity of the mammalian heart by transplanting lab-grown heart muscle cells into the damaged heart. Although this potential therapy is promising, results showed that these lab-grown cells are still immature and cannot communicate properly with the rest of the heart, leading to abnormal contractions called arrhythmias. “Although more research is needed to precisely define how mature these lab-grown heart muscle cells are when treated with LRRC10, it is possible that the increase in maturation will improve their integration after transplantation,” says Jeroen Bakkers, last author of the study. Bakkers continues: “Additionally, current models for cardiac diseases are frequently based on immature lab-grown heart muscle cells. 90% of promising drug candidates found in the lab fail to make it to the clinic and the immaturity of these cells could be one contributing factor for this low success rate. Our results indicate LRRC10 could improve the relevance of these models as well”. LRRC10 could thus have an important contribution to generate lab-grown heart muscle cells that more accurately represent a typical adult human heart, therefore improving the chances of developing successful new treatments against cardiovascular diseases.
Follow-up study with human heart tissue slices
The study of calcium in the cultured zebrafish heart slices was conducted in the lab of Teun de Boer (Medical Physiology, UMC Utrecht). Here, slices of human heart tissue, obtained after heart surgery, are now also being cultured for research into heart disease. Follow-up research into LRRC10, but also into other factors that play a role in diseases of the heart muscle, is being conducted in these slices. De Boer: "The great advantage of the human slices is that they are very representative of the patient, and we can keep them healthy in culture for more than ten days. As a result, we can use many of the techniques we used in zebrafish to study human disease processes as well." Human heart slices are expected to help bridge the gap between the lab and the clinic even further.
Publication
Interplay between calcium and sarcomeres directs cardiomyocyte maturation during regeneration. Phong D. Nguyen, Iris Gooijers, Giulia Campostrini, Arie O. Verkerk, Hessel Honkoop, Mara Bouwman, Dennis E. M. de Bakker, Tim Koopmans, Aryan Vink, Gerda E. M. Lamers, Avraham Shakked, Jonas Mars, Aat A. Mulder, Sonja Chocron, Kerstin Bartscherer, Eldad Tzahor, Christine L. Mummery, Teun P. de Boer, Milena Bellin, Jeroen Bakkers. Science, 2023.
The study is the result of a collaboration between the Hubrecht Institute, LUMC, AMC, UMC Utrecht and Weizmann Institute. The study was financed with the help of the Dutch Heart Foundation, Dutch CardioVascular Alliance and Stichting Hartekind.
--
Jeroen Bakkers is group leader at the Hubrecht Institute and professor of Molecular Cardiogenetics at the UMC Utrecht
Teun de Boer is associate professor at the Department of Medical Physiology, UMC Utrecht
--
About the Hubrecht Institute
The Hubrecht Institute is a research institute focused on developmental and stem cell biology. Because of the dynamic character of the research, the institute as a variable number of research group, around 20, that do fundamental, multidisciplinary research on healthy and diseased cells, tissues and organisms. The Hubrecht Institute is a research institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), situated on Utrecht Science Park. Since 2008, the institute is affiliated with the UMC Utrecht, advancing the translation of research to the clinic. The Hubrecht Institute has a partnership with the European Molecular Biology Laboratory (EMBL). For more information, visit www.hubrecht.eu.
