Oct 28: Combination of existing drugs efficacious in Sjögren’s syndrome
The drug combination of leflunomide plus hydroxychloroquine was safe and resulted in a good clinical response in patients with primary Sjögren’s syndrome. “The successful outcome of this well-controlled study – the first ever to investigate a combination of existing drugs in this disease - warrants further evaluation in larger clinical trials”, concludes Eefje van der Heijden in her PhD thesis.
To date, for patients with Sjögren’s syndrome no effective treatment is available. A variety of immune cells is involved in various mechanisms causing this chronic inflammatory disease. Past research has focused on anti-rheumatic drugs targeting only one type of immune cell, with generally disappointing results. Eefje van der Heijden (Center for Translational Immunology and Department of Rheumatology and Clinical Immunology, UMC Utrecht) hypothesized that a combination of already existing drugs targeting several immune cell types is necessary for optimal efficacy. In her PhD research, she investigated if a drug combination with complementary properties - leflunomide (LEF, 20 mg) and hydroxychloroquine (HCQ, 400 mg) - might fulfill this requirement.
Combination treatment efficacious
In a placebo-controlled, randomized, double-blind clinical trial performed at UMC Utrecht and recently published in Lancet Rheumatology, Eefje van der Heijden and colleagues indeed demonstrated that the LEF-HCQ combination significantly reduced disease activity, as measured by ESSDAI score (see Figure), without occurrence of serious adverse events.
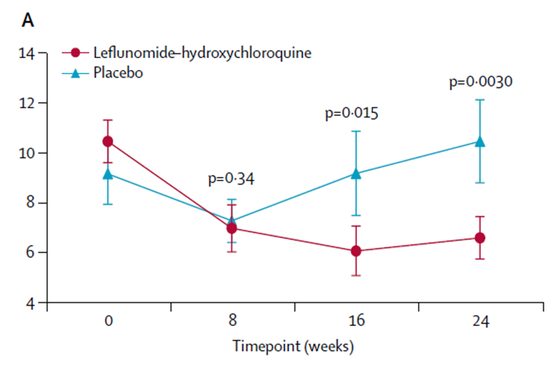
Figure: Disease activity index scores (ESSDAI) over 24 weeks treatment with LEF-HCQ and placebo
Furthermore, in this PhD research the observed clinical improvement as observed with LEF-HCQ was associated with unprecedented significant reductions in patient reported outcomes (pain and fatigue), dryness and decreased markers of inflammation in blood and salivary glands. Moreover, inflammation markers in the blood were shown to accurately predict whether a patient was likely to respond to therapy. Currently, a larger clinical trial is in preparation to further investigate this promising treatment option.
Sjögren’s syndrome
Primary Sjögren’s syndrome is a chronic autoimmune disease characterized by lymphocytic infiltration and functional impairment of exocrine glands. It affects approximately 0.5 percent of the population, mainly women. Patients experience severe dryness of eyes and mouth, and depending on the presence of other sites of inflammation, also may have complaints due to inflammation of a specific organ system such as skin, lungs or kidneys. Furthermore, patients are at increased risk of developing non-Hodgkin lymphoma.
PhD defense
Eefje van der Heijden (1985, Vlijmen) defended her PhD thesis on October 27, 2020 at Utrecht University. The title of her thesis was “Repurposing established drugs for new indications: leflunomide and hydroxychloroquine combination therapy for Sjögren’s syndrome”. Supervisor was prof. dr. Timothy Radstake and co-supervisors were dr. Joel van Roon (Center for Translational Immunology and Department Rheumatology and Clinical Immunology, UMC Utrecht) and dr. Aike Kruize (Department of Rheumatology and Clinical Immunology, UMC Utrecht). Eefje van der Heijden was a finalist in the Erik Hack Publication Award 2020 contest (best manuscript by a PhD candidate as first author) of strategic program Infection & Immunity at UMC Utrecht. She also was a finalist for the NVSP (Dutch association for Sjögren patients) thesis award. Eefje currently works as a resident in internal medicine at the Jeroen Bosch Hospital in ‘s Hertogenbosch (the Netherlands).
