Group Ausems

Both the FOCUS and DISCOVER study focus on mainstream genetic testing pathways for cancer patients. The FOCUS study consists of two studies, one for ovarian cancer and one for breast cancer. The DISCOVER study focuses on prostate cancer. In a mainstream genetic testing pathway, genetic testing is discussed and ordered by the healthcare professional (gynecologist, surgeon, urologist, oncologist, nurse specialist) who is treating the patient, rather than by a clinical geneticist or genetic counselor.
Projecten over mainstreaming van DNA-onderzoek uitklapper, klik om te openen
For breast, ovarian and prostate cancer, a mainstreaming method has been developed and put into use in collaboration with a multidisciplinary team of healthcare providers and patient representatives. We have developed an online knowledge module for each study. This knowledge module explains the heredity of breast, ovarian and prostate cancer and the protocol of the mainstreaming method.
In each participating hospital, a kick-off meeting was organized to explain the study to the healthcare providers involved.
FOCUS uitklapper, klik om te openen
(Future Of Cancer genetic testing as Usual and Standard care) PhD project K. Bokkers
The FOCUS study was funded partially by AstraZeneca and Pfizer and focuses on mainstream genetic testing in patients with ovarian cancer and breast cancer. The aim of these new care pathways was to integrate genetic testing into the routine care of these patients and to facilitate genetic testing for all eligible patients at an early stage after diagnosis.
A mainstream genetic testing pathway for ovarian cancer was implemented in four hospitals and for breast cancer in nine hospitals (see map below). In both studies we evaluated the experiences of healthcare professionals (e.g., time investment, attitudes, self-efficacy, knowledge), of patients (e.g. acceptability, knowledge, discussed topics, decisional conflict, regret), and the number of patients offered genetic testing after implementing these new care pathways. In addition, for the ovarian cancer project, we also evaluated the genetics-related healthcare costs.
The FOCUS study was partly funded by AstraZeneca and Pfizer and focuses on mainstreaming DNA research in patients with ovarian and breast cancer. The aim of these new practices was to integrate DNA research more into the standard of care for these patients and to make access to DNA research easier for all eligible patients.
A mainstreaming method was set up in four hospitals for ovarian cancer and in nine hospitals for breast cancer (see figure below). In both studies, we assess the experience of healthcare providers (e.g. time investment, attitude towards mainstreaming, effectiveness, knowledge), experience of pat patients (e.g. acceptability, knowledge, discussed underwork, choice stress, choice problem), and number of patients who have received DNA research after using the mainstreaming method. In addition, for the ovarian cancer study, we also assess the genetics-related healthcare costs.
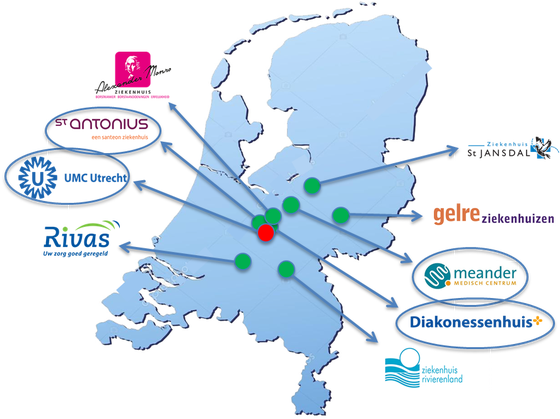
FOCUS study: All hospitals participating in the FOCUS-breast cancer study and the hospitals participating in the FOCUS-ovarian cancer study (only blue encircled)
DISCOVER uitklapper, klik om te openen
(Detecting Increased Susceptibility for Cancer in relatives by Offering genetic Variant Evaluation as Regular health care) PhD project M. Vlaming
The DISCOVER study is funded by KWF Kankerbestrijding and focuses on mainstream genetic testing in patients with prostate cancer. The goal of this new procedure is to find new families with a genetic predisposition for cancer. The DISCOVER study is set-up in collaboration with Radboudumc and the Antoni van Leeuwenhoek hospital. Healthcare professionals of 15 hospitals are participating in the study.
We will evaluate this new procedure on the occurrence of pathogenic variants in the patients and on efficacy. We will look at the experience of healthcare professionals (think about their time investment) and the experience of the patient. We aim to include 800 patients with metastatic prostate cancer. Patients can be included up to September 2023.
Erfo4all project uitklapper, klik om te openen
Equal access to breast cancer genetic counseling and testing, development and implementation of a health literacy training program for surgical oncologists and specialized nurses. PhD project J van der Giessen- van der Doelen
Genetic testing is important for breast cancer patients at risk of carrying a mutation in a breast cancer gene, so that they can be offered the best treatment and take preventive measures if the cancer is hereditary. Jeanine van der Giessen, under the supervision of the department of genetics UMC Utrecht, Nivel and Amsterdam UMC, conducted research on equal access to breast cancer genetic testing.
Her research shows that patients with a lower education level and patients with a migrant background have less access to genetic testing for (breast) cancer. Patients' level of health literacy is crucial in this. These are skills that patients need to obtain, understand, assess and apply health information. Clearly explaining genetic testing to patients with limited health literacy is difficult. If the healthcare professional does not discuss it, it often means that patients are not referred for genetic testing.
Jeanine van der Giessen's PhD study describes the development and evaluation of a training program (Erfo4all) for surgical oncologists and specialized nurses. The aim was to reduce differences in referral to breast cancer genetic testing. The study showed that after completion of the training program, healthcare professionals had increased awareness of the problem of limited health literacy and more self-confidence to communicate effectively, but the number of referred patients with limited health literacy or a migrant background did not change. However, the average age at which patients were referred was lower. This is important, because the risk of hereditary breast cancer is higher for younger women with breast cancer. Plain language when discussing breast cancer genetic testing is necessary to promote equal access.
The erfo4all project was funded by Pink Ribbon.
Jeanine van der Giessen defended her thesis on September 28th, 2021.
HEBON (Hereditary Breast and Ovarian Cancer Netherlands) uitklapper, klik om te openen
Hebon is a large-scale national study of families at increased risk of breast and ovarian cancer. The study is a multidisciplinary collaboration of all departments of Clinical Genetics/Familial Tumors in the Netherlands. All individuals who have ever had genetic testingfor the hereditary breast cancer genes are eligible to participate in this study.
The primary goal of the Hebon study is to facilitate research among families at increased risk of breast and ovarian cancer focusing in particular on:
1. Identifying new genetic risk variants
2. Estimating the risk of cancer (breast, ovary and other forms) within the participating families.
3. Investigating genetic and hormonal/lifestyle factors on the cancer risk.
4. Developing risk models for breast and ovarian cancer including both genetic and non-genetic factors.
5. Studying whether the outcome of cancer treatment depends on genetic susceptibility.
6. Development of prognostic models for breast and ovarian cancer, including genetic factors,
tumor characteristics and various treatments.
Hebon Resource is funded by ZonMw, KWF Dutch Cancer Society and Dutch Research Council/ZonMw. Website: http://www.hebon.nl
Early detection of pancreatic cancer in high-risk individuals (FPC-II study)
Pancreatic carcinoma has a relatively poor prognosis and is ranked among the top five causes of cancer-related mortality in Western populations. A major cause of this high mortality is the late presentation of symptoms, as a result of which less than 20% of all PC patients present with a localized disease and thus qualify for surgical resection.
Screening of individuals at high risk of developing pancreatic ductal adenocarcinoma may lead to timely detection of non-invasive precursor lesions and early-stage asymptomatic cancer. However, such surveillance has not yet been proven to effectively reduce morbidity and mortality. That is why a longitudional study has been set up in which four academic centers (the Erasmuc MC, AMC, UMCG, and UMC Utrecht) and the NKI /AvL participate. Within the UMC Utrecht, the Departments of Genetics and Gastroenterology (Prof. F. Vleggaar) together ensure yearly surveillance for participants.
Individuals with a detected hereditary > increased risk of developing pancreatic cancer are invited to participate in this study. The screening includes MRI (Magnetic Resonance Imaging) and EUS (Endoscopic Ultra Sound) at baseline, followed by annual EUS. In addition, annual blood collection takes place.
With this study, we hope to detect high-grade premalignant lesions and pancreatic cancer at an early stage, with the aim of reducing mortality and morbidity.
